#3025: Phase Change
Permalink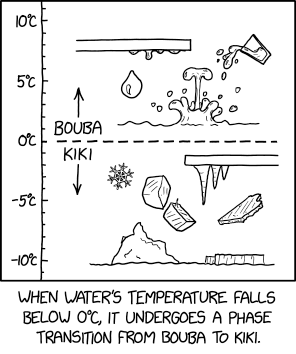
Transcript
[A vertical graph is shown, indicating temperature in degrees Celsius, with a horizontal dotted line at zero degrees and positive temperatures above it. The graph has labels against gradation marks for -10, -5, 0, 5 and 10 °C, a mark for each other whole degree present and extends to meet the top and bottom border of the graph's frame, at approximately ±11.5°C. The area above the dotted line is filled with several illustrations of liquid water in various forms: dripping down from a surface, forming a droplet, pouring from a glass, splashing onto a surface of water, etc. This area is labeled "Bouba" with an arrow pointing up. The area below the dotted line is filled with illustrations of ice in various forms, namely icicles hanging from a surface, two ice cubes (one with a small ice spike), a snowflake, a thin piano-shaped piece of ice and a thicker sheet of ice on water along with an iceberg. This area is labeled "Kiki" with an arrow pointing down.]
[Caption below the panel:]
When water's temperature falls below 0°C, it undergoes a phase transition from bouba to kiki.
(Sourced from explainxkcd.com)
Title text:Even when you try to make nice, smooth ice cubes in a freezer, sometimes one of them will shoot out a random ice spike, which physicists ascribe to kiki conservation.